https://springvalleypediatrics.net/wp-content/uploads/2025/02/Headshot2022-scaled.jpg
2560
1707
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2025-02-12 12:45:242025-02-12 13:22:40Welcome Dr. Lily Joseph
https://springvalleypediatrics.net/wp-content/uploads/2023/10/The-Common-Cold.jpg
1080
1080
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2023-10-08 21:28:262025-02-12 12:58:31The Common Cold
https://springvalleypediatrics.net/wp-content/uploads/2023/09/RSV-and-the-new-vaccine-2.jpg
1080
1080
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2023-09-03 16:37:172025-07-25 15:08:33RSV and the new monoclonal antibody injection
https://springvalleypediatrics.net/wp-content/uploads/2023/03/Covid.jpg
792
612
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2023-03-13 17:32:162024-03-19 21:32:41Dr. Sexter in The New England Journal of Medicine
https://springvalleypediatrics.net/wp-content/uploads/2021/11/In-the-news.png
3000
3000
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-11-14 22:07:512024-03-19 21:32:35Spring Valley Pediatrics in the News
https://springvalleypediatrics.net/wp-content/uploads/2021/11/IMG_6859.jpg
1700
910
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-11-05 09:54:392023-09-03 16:44:02FAQ: Pfizer Vaccine for Ages 5-11
https://springvalleypediatrics.net/wp-content/uploads/2021/11/Copy-of-DID-YOU-GET-THE-JJ-COVID-19-VACCINE.jpg
1080
1080
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-11-01 17:16:182023-09-03 16:44:11UPDATE: COVID-19 Vaccine for Ages 5-11
https://springvalleypediatrics.net/wp-content/uploads/2021/05/Sexter-Screen-Shot-scaled.jpg
1328
2560
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-05-04 12:27:062024-03-19 21:32:18Dr. Sexter on WUSA9
https://springvalleypediatrics.net/wp-content/uploads/2021/04/SUMMER-CAMPS-2021.jpg
1080
1080
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-04-05 15:46:172023-09-03 16:44:41Summer Camps 2021
https://springvalleypediatrics.net/wp-content/uploads/2021/03/IMG_2165-scaled.jpg
2560
1920
Jessica Long
https://springvalleypediatrics.net/wp-content/uploads/2017/01/Spring-Vallery-Pediatrics-300x93.png
Jessica Long2021-03-10 17:03:562023-09-03 16:44:49Breastfeeding and the COVID-19 Vaccine
 We are very excited to announce that Dr. Lily Joseph will be joining Spring Valley Pediatrics this summer.
We are very excited to announce that Dr. Lily Joseph will be joining Spring Valley Pediatrics this summer.



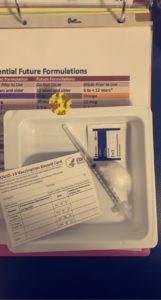

 Dr. Sexter got to talk to WUSA9 about the COVID-19 vaccine trial for kids.
Dr. Sexter got to talk to WUSA9 about the COVID-19 vaccine trial for kids.  With the COVID-19 vaccine becoming more available to the general population, many families have reached out with questions. Is it safe to receive it while breastfeeding? If so, does it offer any protection to the baby?
With the COVID-19 vaccine becoming more available to the general population, many families have reached out with questions. Is it safe to receive it while breastfeeding? If so, does it offer any protection to the baby?